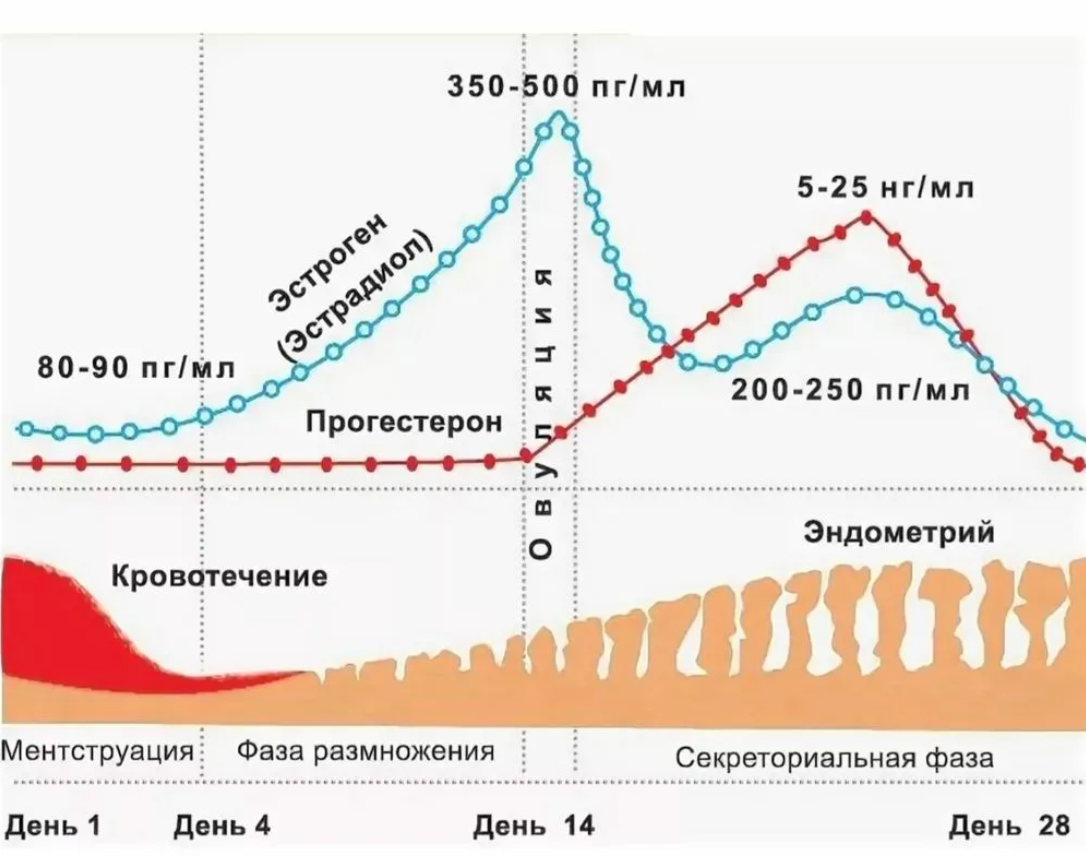
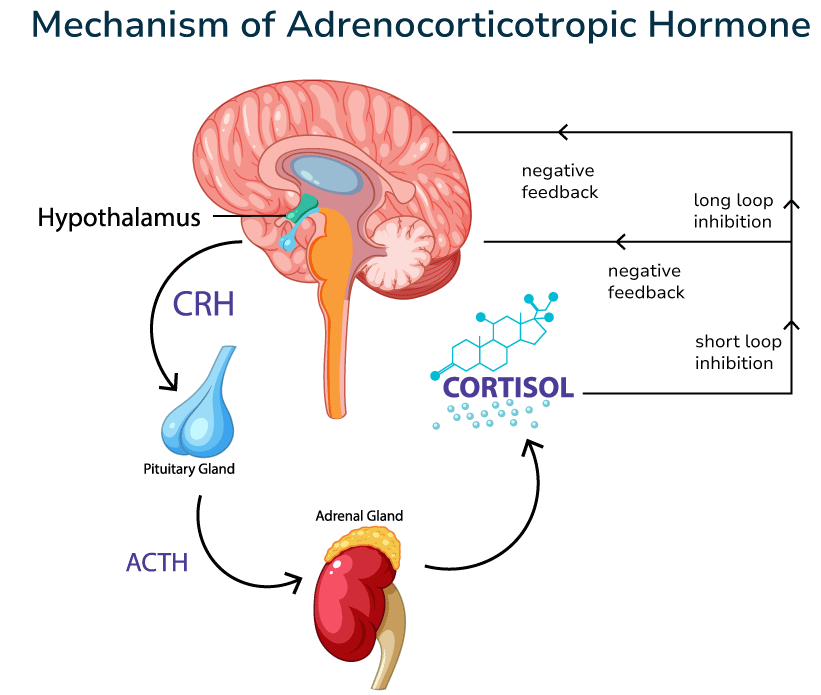
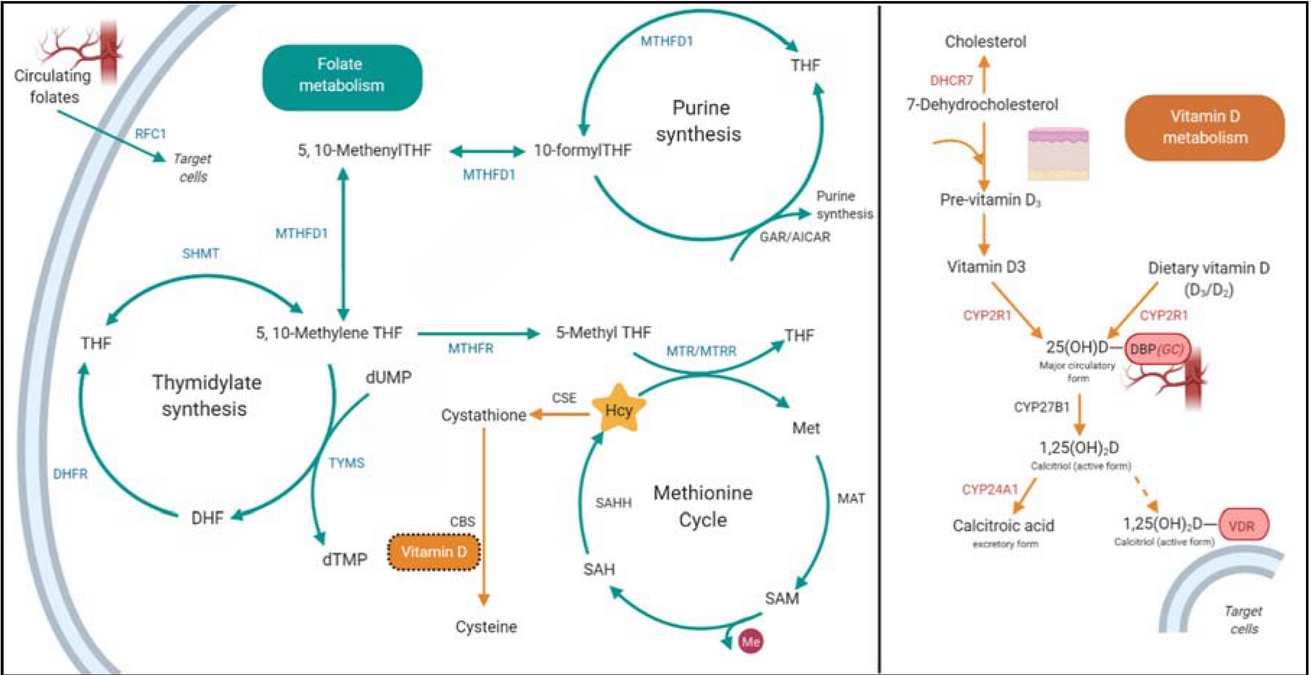
Coenzyme Q10 (Ubiquinone) — it is a vitamin-like substance that plays a key role in the production of energy (ATP) in the mitochondria and the protection of cells from oxidative stress.
Main functions:
- Energy metabolism -participates in the respiratory chain of mitochondria, helping to synthesize ATP (especially important for the heart, brain, and muscles).
- Antioxidant protection -neutralizes free radicals, prevents cell damage.
- Heart support -improves myocardial contractility, reduces the risk of atherosclerosis.
- Slowing down aging – CoQ10 levels decrease with age.
- Immune system -increases the activity of phagocytes.
Symptoms of CoQ10 deficiency
A deficit occurs when:
- Over the age of 40 (natural decrease in synthesis).
- Taking statins (cholesterol-lowering drugs block CoQ10 synthesis).
- Chronic diseases (heart failure, diabetes, Parkinson’s disease).
Signs of a shortage:
✔ Chronic fatigue, weakness.
✔ Muscle achesand cramps (especially when taking statins).
Shortnessof breath, arrhythmia (due to decreased energy in the heart muscle).
✔ Memory loss, cognitive impairment.
✔ High blood pressure.
Symptoms of excess CoQ10
Overdose is extremely rare, as CoQ10 is non-toxic. Possible effects when taking large doses (>300 mg / day):
- Nausea, diarrhea.
- Headache, insomnia.
- Decreased appetite.
Note: A natural excess is not possible – the excess is excreted in the bile.
CoQ10 standards in analyses
CoQ10 levels are measured in blood plasma or lymphocytes (a more accurate method).
| Parameter | Standard | Optimal level |
|---|---|---|
| Blood plasma | 0.5-1.5 mcg / ml | >1.0 mcg / ml |
| Lymphocytes | 30-150 nmol / mg of protein | >50 nmol / mg |
When is the test scheduled?
- With muscle weakness while taking statins.
- For heart failure, migraines.
- To assess the antioxidant status.
How to increase CoQ10?
- Food:
- Fatty fish (herring, sardines).
- Beef, chicken heart.
- Nuts, seeds, spinach.
- Supplements (as directed by your doctor):
- Ubiquinone (the usual form) – 100-200 mg / day.
- Ubiquinol (reduced form– — better absorbed, especially after 40 years.
- Reducing risk factors:
- Quitting smoking (accelerates oxidative stress).
- Blood sugar control (diabetes lowers CoQ10).
Conclusion
CoQ10 is a vital component for energy and antioxidant protection.
Deficiency is manifested by fatigue, muscle weakness, and heart problems.
, Blood norm: 0.5-1.5 mcg / ml (plasma), > 50 nmol / mg (lymphocytes).
Supplements are effective for proven deficiencies or statin use.
Example: If the CoQ10 level is < 0.5 mcg / ml, it is recommended to take 100-200 mg/day in the form of ubiquinol.