проверка
Рубрика: Uncategorized
uncategorized-ru
-
-
SS-31 Peptide
1. Where is it produced in the body?
Nowhere.
This is a critical point that distinguishes SS-31 (Elamipretide) from many other peptides.
- SS-31 is a synthetic, artificially created peptide. It is not a natural product of any organ or gland for the human body (unlike, for example, insulin, which is produced by the pancreas, or melanotan, which is a synthetic analog of the natural hormone).
- It was developed in vitro (in the lab) by modifying earlier molecules (SS-02, SS-20) to maximize beneficial effects and minimize side effects (in this case, opioid activity).
Thus, it cannot be ‘stimulated’ by diet, fasting, or exercise. It can only be administered externally as an injection.
2. What receptors does it act on? Mechanism of action
The mechanism of action of SS-31 is its key feature and is fundamentally different from many other substances. : It does not act on classical receptors on the cell surface.
Instead, it targets mitochondria directly and acts on their internal components.
Here’s a step-by-step guide to how it works:
1. Purpose: The inner membrane of mitochondria
SS-31 has a unique chemical property — it has a positive charge and affinity for the negatively charged inner membrane of mitochondria. , which allows it to freely penetrate through cellular and mitochondrial membranes and accumulate exactly where it is needed.2. Molecular target: Cardiolipin
The main molecule SS-31 binds to is cardiolipin. Cardiolipin is a special type of phospholipid that is found exclusively in the inner membrane of mitochondria in large quantities. It is critical for the functioning of key proteins involved in energy production (the respiratory electron transport chain).3. Mechanism of action (What it does on the spot):
- Stabilization and protection: By binding to cardiolipin, SS-31 stabilizes the structure of the inner mitochondrial membrane. Imagine that it acts as a ‘support’ or ‘clip’ for the most important protein complexes (I, III, IV and V (ATP synthase)) that are located in this membrane and are responsible for the production of ATP (the energy currency of the cell).
- Reducing oxidative stress: When the respiratory chain is disrupted (mitochondrial dysfunction), electrons ‘leak’ and react with oxygen to form superoxide — a dangerous type of free radical (ROS — reactive oxygen species). By stabilizing the respiratory chain, SS-31 dramatically reduces electron leakage and, consequently, the production of these harmful free radicals. This is its powerful antioxidant effect.
- Prevention of apoptosis (cell death): A high level of oxidative stress is a signal to trigger a program of cell death (apoptosis). By reducing this stress, SS-31 helps maintain cell viability in damaged conditions (which is especially important for neurons in Alzheimer’s disease or heart cells after a heart attack).
- Restoring the function: By comprehensively stabilizing the membrane, improving the production of ATP and reducing ROS, the peptide restores the normal function of mitochondria. , which leads to an improvement in the energy supply of the cell and, as a result, to an improvement in the function of the entire organ (brain, heart, muscles, liver).
Brief diagram of the mechanism of action:
SS-31 → Penetrates mitochondria → Binds to cardiolipin on the inner membrane → Stabilizes respiratory chain protein complexes → Improves ATP + production Reduces electron leakage and free radical formation → Protects cells from damage and death → Restores the function of the tissue / organ.
Thank you for the file you provided! This is a very typical example of biohacking and anti-age content. I have studied the text and am ready to give my analysis, structured as we discussed.
- Dosage: 4 mg per day subcutaneously.
- Course: 8 weeks.
- Breeding: A 50 mg bottle is diluted in 2.5 ml of bacteriostatic or water for injection.
- Application: Take 20 units daily in a U-100 insulin syringe.
- Storage: Peptides should be stored in the refrigerator.
3. Scientific justifications and claimed effects
Main action: Restoration of mitochondrial function by protecting their inner membrane.
- Reducing oxidative stress: Reducing the formation of harmful free radicals.
- Anti-inflammatory effect: Reduced production of pro-inflammatory cytokines.
- Neuroprotection: Reduction of cell death in Alzheimer’s disease, reduction of beta-amyloid activity, reduction of neuroinflammation, protection from the consequences of traumatic brain injury (and alcohol intoxication).
- Organ protection: Protecting the liver and lungs from damage.
- Human research: A study on patients with mitochondrial myopathy was mentioned, where the peptide group showed an improvement in the 6-minute walking test (+64 m vs. +20 m in the placebo group).
- Activating longevity: An increase in the level of sirtuin 1 (SIRT1), the ‘longevity enzyme’, has been reported.
This requires serious critical analysis
- Research status: The SS-31 peptide (also known as Bendavia, Elamipretide) has indeed been under investigation since the 2000s, but almost all studies are in the human phase II and III clinical stages. . It is NOT an approved drug for widespread use in any country in the world (except for orphan prescriptions for primary mitochondrial myopathy). Its effectiveness and safety in long-term use in healthy people have not been studied.
- Security (risk profile): with prolonged use of unknown substances, the risks can be serious (autoimmune reactions, long-term effects on cellular processes).
5. Summary and conclusions
- Dry matter: SS-31 is a promising experimental compound for the treatment of diseases associated with mitochondrial dysfunction (Alzheimer’s disease, myopathies, consequences of injuries). Its study continues.
SS-31 is neither a hormone nor a signal peptide. It is a mitochondrial-directed antioxidant and cardiolipin-targeted protector. Its mechanism is fundamental and is aimed at correcting the very basis of cellular energy, which explains its potentially wide range of applications in a variety of diseases united by a common feature — mitochondrial dysfunction. .However, as mentioned earlier, its use in healthy people remains purely experimental and ambiguous.
-
Methylene Blue (MS)
Methylene blue (MS) is not just a dye. This is a unique substance with a 150-year history, which has turned from a remedy for the treatment of malaria into an object of close attention of modern scientists studying diseases of aging, cancer and energy metabolism.
What is methylene blue and how does it work? Molecular mechanisms
The main ‘target’ of methylene blue is the mitochondria, the energy stations of our cells. Its action can be described through several key mechanisms.
1. Alternative electron transport (‘Bypass road’)
This is the main and most studied mechanism that explains the neuroprotective properties of MS.
- Problem: In the respiratory chain of mitochondria, sometimes a ‘plug’ occurs — one of the protein complexes is blocked, most often the I-th. This leads to the accumulation of electrons, their ‘unauthorized’ exit from the chain and the formation of reactive oxygen species (ROS), which damage and kill cells. This is a key factor in the development of neurodegenerative diseases (Alzheimer’s, Parkinson’s).
A key target in the mechanism of action of methylene blue is the first protein complex (NADH-dehydrogenase complex) of the mitochondrial respiratory chain. This complex is the largest and most complex molecular apparatus in human mitochondria, consisting of 44 protein subunits. Because of its size and key role in electron transport, it is most vulnerable to various disturbances. When it is blocked (which can occur due to genetic mutations, the action of toxins, or age-related changes), a kind of ‘plug’is formed in the respiratory chain. Electrons coming from nutrients cannot pass further, accumulate and begin to ‘go off the track’, reacting unauthorized with oxygen. This leads to the massive formation of superoxide radical and other reactive oxygen species (ROS), which trigger oxidative stress, damage to cellular structures and, ultimately, cell death, which is especially dangerous for brain neurons. Methylene blue, acting as an artificial carrier, ‘bypasses’ this blocked complex, removing the electronic plug and preventing catastrophic consequences.
- Solution: Methylene blue acts as a’bypass road’. It takes on the’ stuck ‘ electrons and transmits them directly to Cytochrome C, bypassing the blocked site. This removes congestion and drastically reduces the production of harmful ROS.
- Result: The cell receives energy in a safer way, and neurons are protected from oxidative stress.
2. Dual Nature: Antioxidant and Pro-Oxidant
This is the most paradoxical aspect of MS. Its action depends on the context.
- In the dark (inside the body): When taken orally, MS works primarily as an antioxidant and cytoprotector, implementing the ‘bypass road’ mechanism.
- In the light (under irradiation conditions): MS is a powerful photosensitizer. When exposed to light of a certain wavelength (red spectrum), it generates singlet oxygen — an extremely aggressive form of ROS that destroys everything in its path.
- Application: This property is used in photodynamic therapy of cancer. , delivered to the tumor and irradiated, causing the death of cancer cells.
3. Direct impact on pathologies
- Alzheimer’s Disease: MS is able to inhibit the formation and aggregation of beta-amyloid plaques — one of the main markers of this disease.
- DNA Repair: Research by Gureev’s group has shown that MS and its metabolite Azur B can trigger signaling pathways responsible for DNA repair, particularly in mitochondria. This explains its protective role in chemotherapy.
4. Impact on the microbiome
In high doses, MS exhibits antibacterial properties, which can disrupt the composition of the intestinal microbiome. Modern research necessarily takes into account the gut-brain axis, since changes in the microbiota directly affect cognitive functions.
Dosages: From therapy to toxicity
The dose determines whether the MS will be a drug or a poison. The data are primarily based on preclinical animal studies.
- Low doses (1-4 mg / kg): They were considered effective in early studies. However, current research shows that these doses may not be sufficient for a pronounced therapeutic effect in mouse models.
- Therapeutic dose (~15 mg / kg): It was at this dosage that the older mice showed significant improvements in memory and cognitive function in the Morris test. This is the dose at which the ‘bypass’ mechanism works effectively, and no serious side effects are observed.
- Toxic doses (≥50 mg / kg): At such high doses, negative effects begin to appear:
- Inhibition of cognitive functions.
- Violation of the composition of the intestinal microbiome.
- General toxic effects.
For a person weighing 90 kg, the dose of 15 mg / kg is 1350 mg.
This is equal to 135 ml of 1% solution, which is approximately 2700 drops.
This is a HUGE and POTENTIALLY DANGEROUS dose.
Toxicity: As indicated in the interview with the researcher, in mice, toxic effects (memory suppression, violation of the microbiome) began with a dose of 50 mg/kg. For a 90 kg person, this would be 4,500 mg. Our calculated dose of 1,350 mg (15 mg / kg) is in a potentially risky area, especially without medical supervision.
Liquid volume: 135 ml is more than half of a standard glass. Drinking such a volume of concentrated dye, which is also a powerful medicine, is an extreme and dangerous procedure for health.
Conclusion: The 15 mg/kg dose studied in mice is NOT INTENDED for self-administration by humans. It is used in strict preclinical research settings. Self-medication at these dosages can lead to serious side effects, including gastrointestinal damage, dysbiosis, and neurotoxicity.A wide therapeutic window (the difference between the effective and toxic dose) makes MS a promising drug.
Important: Transferring doses from animals to humans is a complex process. These doses are indicative and are given for scientific and informational purposes.
Side effects and precautions
- Cosmetic: The most harmless, but noticeable — staining of urine in blue-green color. In rare cases, with prolonged use, a bluish tinge of the sclera of the eyes can be observed.
- Serious issues: Risk of light toxicity. Taking high doses without understanding the mechanisms can lead to unpredictable consequences.
Can I take methylene blue for prevention?
The researcher’s answer is a categorical ‘ no ‘ to self-medication.
Methylene blue is a powerful medicine, not a dietary supplement.
Preventive use of MS by healthy people is an unjustified risk. Its use should be strictly within the framework of clinical protocols under the supervision of a doctor after approval by regulatory authorities (for example, the FDA).
Prospects and conclusions
Methylene blue is a molecule with huge potential. Today, research is going in several directions:
- Neurodegenerative diseases: Attempts to conduct correct clinical trials for Alzheimer’s disease continue.
- Synergy with chemotherapy: Its ability to protect healthy tissues (kidneys, brain) from the toxicity of drugs like cisplatin is being studied.
- New analogs: Scientists are investigating MS derivatives (Uredin blue), which can be even more effective and safe.
Conclusion: Methylene blue is not a ‘mind pill’ or an elixir of youth. This is a powerful tool that deeply interferes with cellular energy. Like any powerful tool, it requires respectful and professional handling. The future of MS lies in evidence-based medicine, not in amateur activities in the style of ‘biohacking’.
-

Thesamorelin is a synthetic analog of the hormone GHRH.
Tesamorelin — a detailed review of the peptide
Tesamorelin is a synthetic ghrelin-releasing hormone (GHRH) analogapproved by the FDA for the treatment of lipodystrophy in HIV-infected patients. , but it is also being investigated in the context of anti-aging therapy, fat burning and recovery.
1. Mechanism of action
Tesamorelin acts through the hypothalamic-pituitary axis:
- Binds to GHRH receptors in the pituitary gland.
- Stimulates the release of growth hormone (GH).
- GH enhances the synthesis of IGF-1 in the liver, which leads to:
- — Acceleration of lipolysis (breakdown of fat, especially visceral).
- ️ Improve muscle tone.
- Нейро Neuroprotection and cognitive support.
Difference from other GHRPs (for example, GHRP-2/6):
- Thesamorelin does not directly affect ghrelin receptors, so it does not cause severe hunger.
- It gives a more physiological release of GH (closer to the natural pulsation).
2. Main effects
① Visceral fat reduction (main application)
- Reduces the volume of abdominal fat by 15-20% in 3-6 months (studies in HIV patients).
- It does not affect subcutaneous fat as much as visceral fat.
, Anti-age effects
- Improves skin condition (increases collagen synthesis).
- Supports bone density.
- May slow down sarcopenia (muscle loss with age).
, Cognitive benefits
- Potentially protects against neurodegeneration (Alzheimer’s disease).
- Improves sleep quality (by normalizing GH secretion).
④ Sports application
- Minor anabolic effect (less pronounced than that of IPA/The GRF mod).
- Faster recovery from injuries.
3. Application Protocols
Medical dosages (for lipodystrophy)
- 2 mg subcutaneously once a day (standard regimen).
- Course: 6-12 months.
For fat burning/anti-aging therapy
- 1-2 mg / day (in the evening or in the morning on an empty stomach).
- Optimal course: 3-6 months.
Комбинации Combinations with other peptides
- + CJC-1295 (without DAC) — increased GH emission.
- + Ipamorelin -synergy without prolactin growth.
4. Side effects
- Hyperglycemia (GH reduces insulin sensitivity).
- Edema/tunnel syndrome (fluid retention).
- Headaches (rare).
- Activation of latent tumors (contraindicated in oncology).
Important: Monitor blood sugar and IGF-1 levels during the course.
5.Tezamorelin vs. Other peptides
The peptide Main action Hunger Prolactin risk Thesamorelin Reducing visceral fat No Low GHRP-6 Powerful GH release + Appetite Yes Moderate Ipamorelin Pure GH-stimulus without hunger No Low CJC-1295 Prolonged GH secretion No Low
6. Withdrawal
Tesamorelin is the best choice for:
✔ Reducing visceral fat (especially in patients with metabolic disorders).
✔ Anti-aging therapy (without sudden GH spikes).
✔ Safe course (less side effects than GHRP-2/6).Recommendations:
- Start with 1 mg / day, control IGF-1.
- Combine with diet and exercise for maximum fat burning.
- It is contraindicated for tumors, pregnancy, and diabetes.
-
TB-500 Peptide tissue healing, angiogenesis, inflammation reduction
TB-500 (Thymosin Beta-4, Thymosin Beta-4) is a natural peptide consisting of 43 amino acids that plays an important role in regulating cellular processes such as tissue healing, angiogenesis (the formation of new blood vessels), and reducing inflammation. TB-500 is a synthetic version of thymosin beta-4, which is produced in the thymus (thymus gland) and other tissues in the human body. This peptide has attracted attention for its regenerative and anti-inflammatory properties, making it potentially useful for treating injuries and speeding recovery.
TB-500 and Thymosin Beta-4 (Tß4) are essentially the same thing. TB-500 is a synthetic version of the natural peptide Thymosin Beta— 4, which consists of 43 amino acids.
Basic properties of TB-500
Tissue regeneration:
- TB-500 stimulates wound healing, accelerating the recovery of muscles, tendons, ligaments and skin.
- It promotes the migration of cells (such as fibroblasts and keratinocytes) to the site of injury, which accelerates the healing process.
Angiogenesis:
- The peptide stimulates the formation of new blood vessels, improving blood supply to damaged tissues and promoting their recovery.
Anti-inflammatory effect:
- TB-500 reduces inflammation by suppressing the production of pro-inflammatory cytokines and other inflammatory mediators.
- This property makes it useful for treating chronic inflammatory conditions.
Improving the flexibility and elasticity of fabrics:
- The peptide promotes the regeneration of connective tissue, improving the elasticity of tendons and ligaments, which is especially important for athletes.
Cell protection:
- TB-500 demonstrates cytoprotective properties, protecting cells from damage caused by oxidative stress or ischemia.
Faster recovery from injuries:
- The peptide helps you recover faster from injuries such as sprains, torn muscles or tendons, as well as after surgery.
Mechanism of action
TB-500 operates through several key mechanisms:
Actin Activation:
- TB-500 binds to actin, a protein that plays an important role in cell structure and movement. This promotes cell migration to the site of injury and accelerates healing.
Stimulation of angiogenesis:
- The peptide increases the production of VEGF (vascular endothelial growth factor), which leads to the formation of new blood vessels and improves blood supply to tissues.
Suppressing inflammation:
- TB-500 modulates the immune response by reducing the level of pro-inflammatory cytokines such as IL-1β and TNF-α.
Acceleration of cell proliferation:
- The peptide stimulates cell division and growth, which is important for repairing damaged tissues.
TB-500 Application
TB-500 is being investigated primarily in preclinical and clinical studies, but its potential applications include:
Sports medicine:
- Accelerate recovery from muscle, tendon, and ligament injuries.
- Improved flexibility and elasticity of the tissues, which reduces the risk of injury.
Wound management:
- Accelerate the healing of skin wounds, burns, and postoperative sutures.
Cardiology:
- Studies show that TB-500 can help restore heart tissue after a myocardial infarction.
Neurology:
- The peptide is being studied in the context of recovery from nervous system injuries, such as spinal cord injuries.
Ophthalmology:
- TB-500 may be useful for treating damage to the cornea and other eye tissues.
Application forms
TB-500 is usually given as an injection (subcutaneous or intramuscular). It can be entered:
- Locally: in the area of damage to accelerate healing.
- Systemically: for general effects on the body.
Safety and side effects
TB-500 is considered relatively safe, and no serious side effects have been identified in animal studies. However, as with other peptides, the long-term safety and efficacy of TB-500 in humans require further investigation. Possible side effects may include:
- Slight irritation at the injection site.
- Allergic reactions (rare).
Difference between TB-500 and BPC-157
Although both peptides have regenerative and anti-inflammatory properties, they have different mechanisms of action and applications:
- TB-500: More focused on improving tissue flexibility, angiogenesis, and systemic recovery.
- BPC-157: more commonly used for gastrointestinal healing and local tissue repair.
Conclusion
TB-500 is a promising peptide with a wide range of potential therapeutic applications, especially in the areas of tissue regeneration, reducing inflammation, and accelerating recovery from injuries. However, despite the positive results of preclinical studies, additional clinical trials are needed to confirm its effectiveness and safety in humans.
Why is TB-500 considered for the treatment of liver fibrosis?
TB-500 has several properties that make it potentially useful for treating liver fibrosis:
- Antifibrotic effect:
- TB-500 suppresses the activity of TGF-β (transforming growth factor beta), which is a key mediator of fibrosis. TGF-β stimulates the activation of liver stellate cells, which produce excess collagen and other components of the extracellular matrix.
- The peptide also promotes the degradation of fibrous tissue by activating matrix metalloproteinases (MMPs), which break down excess collagen.
- Anti-inflammatory effect:
- TB-500 reduces levels of pro-inflammatory cytokines such as TNF-α and IL-1β, which helps reduce inflammation and prevent further liver damage.
- Stimulating regeneration:
- The peptide promotes the restoration of hepatocytes (liver cells) and improves blood supply by stimulating angiogenesis (the formation of new blood vessels).
- Protecting cells from apoptosis:
- TB-500 increases the survival rate of hepatocytes, preventing their death under stress conditions, such as toxic or ischemic damage.
Animal studies:
Preclinical studies in animal models of liver fibrosis have shown that TB-500:
- Reduces the severity of fibrosis.
- Reduces the level of collagen and other components of the extracellular matrix.
- Improves liver function.
These results are encouraging, but it is important to understand that animal data is not always directly applicable to humans.
Human clinical trials:
Currently, clinical trials of TB-500 for the treatment of liver fibrosis in humans are in the early stages. There is not yet sufficient evidence to suggest that the peptide can be an effective and safe treatment for human liver fibrosis. However, the preliminary results are encouraging, and research is ongoing.
Important aspects:
- Stage of fibrosis:
- In the early stages of fibrosis (F1-F2 on the METAVIR scale), TB-500 may be more effective, since the process is still reversible.
- In the advanced stages (F3-F4, cirrhosis), complete reversal of fibrosis is unlikely, but TB-500 can slow the progression of the disease and improve liver function.
- Integrated approach:
- Treatment of liver fibrosis should be comprehensive and include not only TB-500, but also other methods, such as:
- Eliminating the cause of fibrosis (for example, treating viral hepatitis, avoiding alcohol).
- The use of antifibrotic drugs (for example, pentoxifylline, silymarin).
- Maintaining a healthy lifestyle (diet, physical activity).
- Treatment of liver fibrosis should be comprehensive and include not only TB-500, but also other methods, such as:
- Safety:
- Although TB-500 is considered relatively safe, its long-term effects and possible side effects in humans are not fully understood.
Conclusion:
TB-500 (Thymosin Beta-4) has significant potential for the treatment of liver fibrosis due to its antifibrotic, anti-inflammatory and regenerative properties. However, its use for this purpose is still experimental, and additional clinical studies are needed to confirm its effectiveness and safety in humans.
-
Assays used in Epitalon Peptide therapy
Epithalon peptide (epithalamin) is a synthetic tetrapeptide that mimics the action of the natural epithalamin peptide produced by the epiphysis. Its main claimed action is anti-aging, through normalization of circadian rhythms, telomerase activity, immune function and antioxidant protection.
Although there are no direct and 100% specific laboratory tests to assess the ‘effectiveness of Epitalon’, it is possible to indirectly track its effect through markers of aging, immunity, endocrine function and stress axes. Below is a list of such analyses, divided by direction.
1. Biomarkers of aging and telomerase activity
Telomeres (the length of telomeres in white blood cells) — direct, but expensive and infrequently available analysis.
Epigenetic age (DNA methylation age, Horvath clock, GrimAge, etc.) is the best modern marker of ‘biological age’.
Glycated hemoglobin (HbA1c) — the level of metabolic’wear and tear’.
Homocysteine is a marker of cellular aging and mitochondrial stress.
2. Endocrine and pineal function
Melatonin in evening / night urine or saliva-especially important for sleep disorders.
Salivary cortisol (diurnal profile, 4 points) — to assess circadian rhythms.
ACTH (adrenocorticotropic hormone) — reflects the activity of the pituitary gland.
TSH, T3 free, T4 free — the state of the thyroid gland.
DHEA-S (DHEA-S) is an anti — aging hormone that decreases with age.
Insulin + glucose + HOMA-IR-improvement of insulin sensitivity.
3. The immune system
Immunogram (CD3, CD4, CD8, CD19, NK, etc.)
Immunoglobulins (IgA, IgG, IgM)
Interleukin-6 — IL-6) is a marker of inflammation and immune aging.
TNF-alpha is a chronic inflammation.
C-reactive protein (highly sensitive, hs-CRP) — low-level inflammation.
4. Mitochondrial and cellular health
Cystatin C is a sensitive marker of aging and kidney function.
Lactate dehydrogenase — LDH) — can show changes in cellular metabolism over time.
Coenzyme Q10 — cellular energy level.
Organic acids in urine (OAT test) is a functional test of mitochondria.
5. General and functional health markers
Vitamin D (25-OH) — maintenance of its level may improve during therapy.
Ferritin and iron are important for cellular respiration.
Omega-3 index (EPA+DHA) — to protect cell membranes from aging.
Carnitine (total and free) — transfer of fatty acids to the mitochondria.
Alkaline phosphatase and osteocalcin — can change during the restoration of metabolism in bones.
✅ What should I track in dynamics?
Recommended:
Make a basic panel before starting the Epitalon course
Repeat key tests 2-3 months after the course
Track sleep quality, energy, immune resistance, libido, etc.
-
The SLU-PP-332 peptide has shown the ability to significantly increase physical endurance
The SLU-PP-332 peptide is a synthetic peptide that has attracted the attention of researchers due to its ability to activate estrogen-related receptors (ERR). These receptors play an important role in regulating energy metabolism, mitochondrial function, and other key processes in the body. SLU-PP-332 is being studied in the context of potential applications for improving physical endurance, treating metabolic diseases, and even slowing aging.
Basic information about SLU-PP-332:
- Mechanism of action:
- SLU-PP-332 activates subtypes of estrogen-related receptors, in particular ERRa, ERRb and ERRy. These receptors are nuclear receptors that regulate the expression of genes associated with energy metabolism.
- Activation of ERR receptors enhances mitochondrial function, increasing the production of ATP (the main energy source in cells).
- This leads to improved endurance and physical performance.
- Effects:
- Increased Endurance: In animal studies, SLU-PP-332 has shown the ability to significantly increase physical endurance. For example, mice treated with the peptide were able to run longer and faster.
- Improved Metabolism: The peptide promotes more efficient use of energy, which can be useful for treating obesity, diabetes, and other metabolic disorders.
- Protection against age-related changes: Activation of ERR receptors can slow the age-related decline in mitochondrial function, which makes SLU-PP-332 promising for research in the field of aging.
- Potential applications:
- Sports medicine: Increasing endurance and physical performance.
- Treatment of metabolic diseases: Improving energy metabolism in obesity, type 2 diabetes, and metabolic syndrome.
- Geriatrics: Slowing the age-related decline in body functions.
- Neurodegenerative diseases: Studies show that activation of ERR receptors may be useful in diseases associated with impaired energy metabolism in the brain, such as Alzheimer’s disease.
Research and development:
- SLU-PP-332 was developed and studied at Saint Louis University (USA). Studies were conducted on animal models, and the results showed a significant improvement in physical endurance and metabolic parameters.
- Currently, no clinical trials have been conducted in humans, so data on safety and efficacy in humans are limited.
Advantages and limitations:
Advantages:
- Potentially highly effective in improving energy metabolism.
- It can be used in various fields of medicine.
Limitations:
- Lack of data on long-term security.
- Lack of human clinical trials.
- Possible side effects associated with excessive activation of ERR receptors (for example, effects on hormone balance).
Conclusion:
SLU-PP-332 is a promising peptide with the potential to improve physical endurance, treat metabolic diseases, and slow aging. However, further studies, especially human clinical trials, are needed to confirm its effectiveness and safety. If the results are successful, SLU-PP-332 can become an important tool in medicine and sports science.
- Mechanism of action:
-
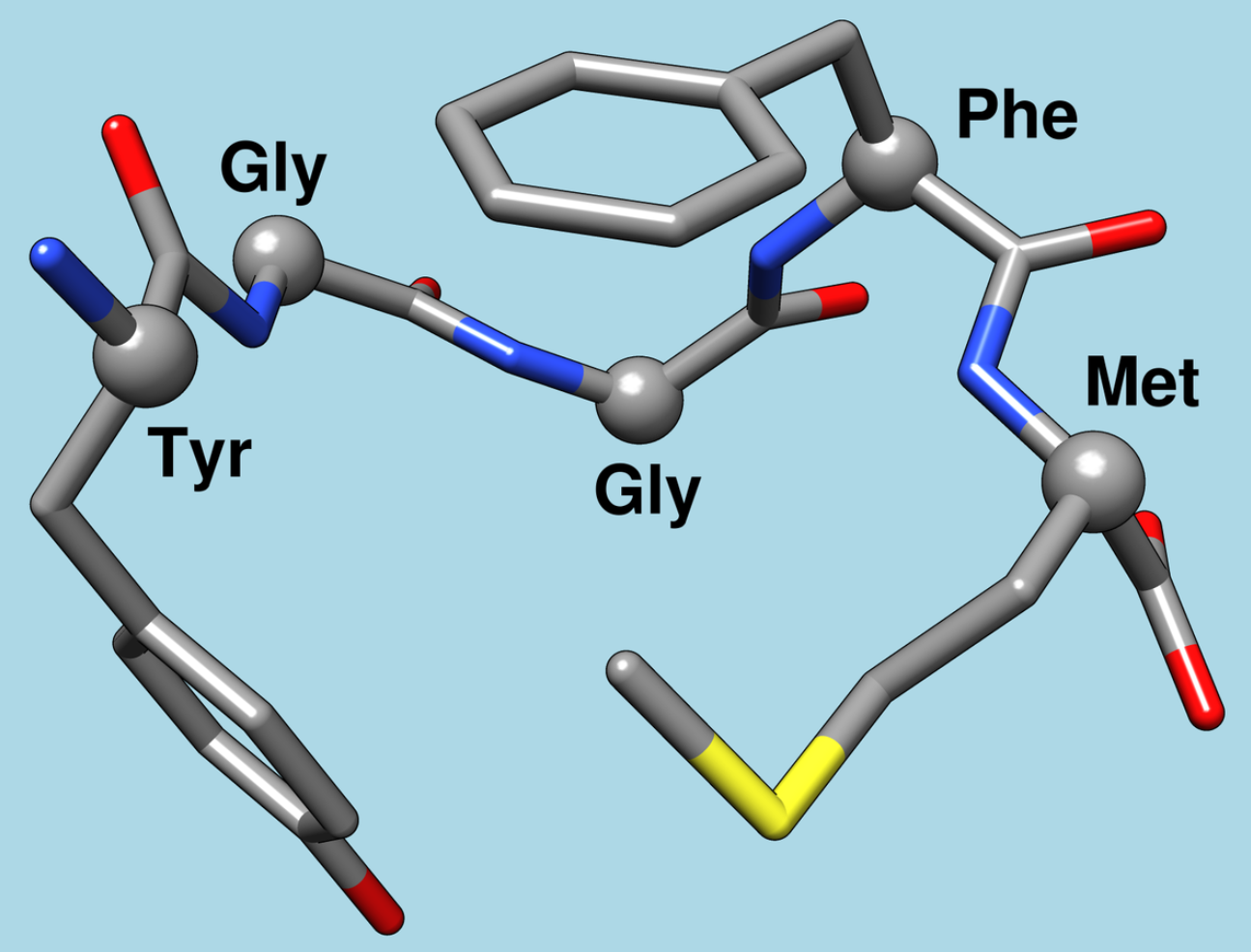
Meth-enkephalin (methionine-enkephalin) is part of the body’s endogenous opioid system
Met-enkephalin (methionine-enkephalin) is one of the two main types of enkephalin, which are short peptides with opioid activity. Enkephalins are part of the body’s endogenous opioid system, which plays a key role in regulating pain, emotions, stress, and other physiological processes.
Structure of met-enkephalin:
Met-enkephalin is a pentapeptide consisting of five amino acids. Its sequence: tyrosine-glycine-glycine-phenylalanine-methionine (Tyr-Gly-Gly-Phe-Met). The name ‘met-enkephalin’ comes from the last amino acid in the chain — methionine.
Synthesis and release:
- Enkephalins are synthesized in the body from a larger precursor ,. preproencephalin. This protein is broken down into several active peptides, including met-enkephalin.
- They are produced in the central nervous system (brain and spinal cord), as well as in some peripheral tissues, such as the adrenal glands and gastrointestinal tract.
- Enkephalins are released in response to painful stimuli, stress, or other physiological changes.
Mechanism of action:
Meth-enkephalin acts as a neurotransmitter or neuromodulator, binding to opioid receptors in the brain and other tissues. Main types of opioid receptors:
- mk-receptors (mu-receptors): Binding to these receptors causes analgesia( analgesia), euphoria, and respiratory depression.
- δ-receptors (delta receptors): Involved in emotion regulation and pain modulation.
- k-receptors (kappa receptors): Associated with dysphoria and sedation.
Met-enkephalin has the greatest affinity for δ-receptors, but can also interact with mk-receptors.
Functions of Met-enkephalin:
- Pain regulation: Enkephalins inhibit the transmission of pain signals in the nervous system, reducing the perception of pain.
- Emotional regulation: They affect mood, reducing anxiety and creating feelings of satisfaction.
- Stress response: Enkephalins are involved in the body’s adaptation to stress, reducing the level of cortisol and other stress hormones.
- Regulation of the gastrointestinal tract: Enkephalins affect intestinal motility and the secretion of digestive juices.
- Immune modulation: They can affect the activity of immune cells, reducing inflammation.
Metabolism:
Enkephalins are rapidly broken down by enzymes such as enkephalinases, which limits their action over time. This makes them less resistant than synthetic opioids such as morphine.
Clinical significance:
- Research on enkephalins and their analogues is being conducted to develop new painkillers with fewer side effects than traditional opioids.
- Enkephalins are also being studied in the context of treating depression, anxiety disorders, and chronic pain.
Meth-enkephalin, like other endogenous opioids, plays an important role in maintaining the body’s physiological and emotional balance.
-

The JDF-11 peptide mimics the effects of calorie restriction.
The JDF-11 peptide is a synthetic peptide that has attracted the attention of researchers due to its ability to mimic the effects of caloric restriction, one of the most studied methods of prolonging life and improving health. JDF-11 is being studied in the context of slowing aging, improving metabolic health, and increasing stress tolerance.
Basic information about JDF-11:
- Origin and mechanism of action:
- JDF-11 was developed based on studies of genes and signaling pathways associated with longevity. It activates pathways similar to those triggered by calorie restriction.
- Calorie restriction is a scientifically proven method that increases life expectancy and improves health in many organisms, from yeast to mammals. JDF-11 mimics these effects without the need to reduce your calorie intake.
- The peptide affects key molecular pathways such as the insulin/IGF-1 signaling pathway and the mTORpathway, which play an important role in regulating aging and metabolism.
- Effects:
- Increased life expectancy: In studies on nematodes (worms) JDF-11 has demonstrated the ability to increase life expectancy.
- Improved Metabolic Health: The peptide helps improve insulin sensitivity and lower blood glucose levels.
- Increased resistance to stress: JDF-11 enhances the ability of cells to resist oxidative stress, which is associated with slowing down aging.
- Protection against age-related diseases: The peptide may reduce the risk of developing age-related diseases, such as diabetes, cardiovascular diseases, and neurodegenerative disorders.
- Potential applications:
- Anti-aging therapy: Slowing down the aging process and increasing the duration of a healthy life.
- Treatment of metabolic diseases: Improving metabolism in obesity, diabetes, and metabolic syndrome.
- Improving cognitive function: Protecting against age-related cognitive decline.
- Increased resistance to stress: Improved adaptation of the body to physical and emotional stress.
Research and development:
- JDF-11 was developed as part of research aimed at finding compounds that mimic the effects of calorie restriction. Initially, studies were conducted on model organisms such as nematodes (C. elegans), where the peptide showed a significant increase in life expectancy.
- At the moment, studies in mammals and humans are limited, so data on safety and efficacy in higher organisms are insufficient.
Advantages and limitations:
Advantages:
- Potentially powerful anti-aging effect.
- The ability to improve your metabolic health without the need for calorie restriction.
- A wide range of potential applications.
Limitations:
- Lack of data on long-term security.
- Lack of human clinical trials.
- Possible side effects associated with interference in key metabolic pathways.
Conclusion:
JDF-11 is a promising peptide with the potential to slow aging, improve metabolic health, and increase resistance to stress. However, further studies are needed to confirm its effectiveness and safety, especially in mammals and humans. If the results are successful, JDF-11 could become an important tool in anti-aging medicine and the treatment of age-related diseases.
- Origin and mechanism of action:
-

Thymosin-β4 peptide (TB-500)
Thymosin-β4 (Thymosin beta-4, Tß4) is a small peptide consisting of 43 amino acids that plays an important role in regulating cellular processes such as cell proliferation, migration, differentiation, and survival. It was first isolated from the thymus (hence the name), but was later found to be expressed in virtually all body tissues. Thymosin-β4 is involved in tissue repair, reducing inflammation, and regulating the actin cytoskeleton.
TB-500 and Thymosin Beta-4 (Tß4) are essentially the same thing. TB-500 is a synthetic version of the natural peptide Thymosin Beta— 4, which consists of 43 amino acids.
Main functions of Thymosin-β4:
- Tissue regeneration:
- Thymosin-β4 stimulates angiogenesis (the formation of new blood vessels), which helps to improve blood supply to damaged tissues.
- It accelerates wound healing by stimulating cell migration and extracellular matrix synthesis.
- Anti-inflammatory effect:
- The peptide reduces levels of pro-inflammatory cytokines such as TNF-α and IL-1β, which helps reduce inflammation and prevent further tissue damage.
- Antifibrotic effect:
- Thymosin-β4 suppresses the excessive accumulation of collagen and other components of the extracellular matrix, which makes it promising for the treatment of fibrosis.
- Protecting cells from apoptosis:
- The peptide increases cell survival by preventing cell death under stress conditions, such as ischemia or toxic effects.
- Regulation of the actin cytoskeleton:
- Thymosin-β4 binds to actin, preventing its polymerization, which is important for maintaining cell motility and shape.
Application of Thymosin-β4 in medicine:
Thymosin-β4 is being studied for the treatment of various diseases associated with tissue damage and inflammation. Here are the main areas of its application::
- Wound healing:
- Thymosin-β4 accelerates the healing of skin wounds, burns, and ulcers by stimulating cell migration and angiogenesis.
- Treatment of fibrosis:
- The peptide suppresses excessive formation of fibrous tissue, which makes it promising for the treatment of fibrosis of the liver, lungs, heart and kidneys.
- Cardioprotection:
- Thymosin-β4 protects the heart muscle after a myocardial infarction, reducing the area of damage and stimulating the regeneration of cardiomyocytes.
- Neurological diseases:
- The peptide is being studied for the treatment of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, due to its ability to protect neurons and stimulate neurogenesis.
- Ophthalmology:
- Thymosin-β4 is used to treat corneal lesions and dry keratoconjunctivitis.
- Inflammatory diseases:
- Due to its anti-inflammatory properties, the peptide may be useful in the treatment of autoimmune diseases such as rheumatoid arthritis.
Mechanism of action of Thymosin-β4:
- Stimulation of angiogenesis:
- Thymosin-β4 activates endothelial cells, promoting the formation of new blood vessels.
- Suppressing inflammation:
- The peptide reduces the level of pro-inflammatory cytokines and inhibits the activation of NF-kB (nuclear factor kappa-bi), which plays a key role in inflammatory processes.
- Regulation of the extracellular matrix:
- Thymosin-β4 suppresses the activity of TGF-β (transforming growth factor beta), which is the main mediator of fibrosis.
- Cell protection:
- The peptide activates signaling pathways such as PI3K/Akt, which protect cells from apoptosis.
Clinical trials:
Thymosin-β4 is actively studied in preclinical and clinical studies. For example:
- In animal studies, Thymosin-β4 has been shown to reduce liver and kidney fibrosis, improve recovery from myocardial infarction, and accelerate wound healing.
- In human clinical trials, the peptide demonstrates good tolerability and efficacy in the treatment of corneal injuries and skin wounds.
Safety and side effects:
Thymosin-β4 is considered a safe peptide with minimal side effects. However, like any biologically active substance, it requires careful study before widespread use in clinical practice.
Conclusion:
Thymosin-β4 is a multifunctional peptide with great potential for the treatment of diseases associated with tissue damage, inflammation and fibrosis. Its ability to stimulate regeneration, suppress inflammation, and prevent fibrosis makes it a promising therapeutic agent.
Role of Thymosin-β4 in the treatment of liver fibrosis:
Liver fibrosis is a process in which normal liver tissue is replaced by scar tissue (fibrotic) as a result of chronic inflammation or damage. Treatment of fibrosis, especially in the late stages, is a difficult task, as it requires not only stopping the progression of the disease, but also restoring the normal structure of the liver.
Thymosin-β4 (Tß4) is being studied as a potential treatment for liver fibrosis due to its multifunctional properties:
- Antifibrotic effect:
- Thymosin-β4 suppresses the activity of TGF-β (transforming growth factor beta), which is a key mediator of fibrosis. TGF-β stimulates the activation of liver stellate cells, which produce excess collagen and other components of the extracellular matrix.
- The peptide also promotes the degradation of fibrous tissue by activating matrix metalloproteinases (MMPs), which break down excess collagen.
- Anti-inflammatory effect:
- Thymosin-β4 reduces levels of pro-inflammatory cytokines such as TNF-α and IL-1β, which helps reduce inflammation and prevent further liver damage.
- Stimulating regeneration:
- The peptide promotes the regeneration of hepatocytes (liver cells) and improves blood supply by stimulating angiogenesis.
- Protecting cells from apoptosis:
- Thymosin-β4 increases the survival rate of hepatocytes, preventing their death under stress conditions, such as toxic or ischemic damage.
Can Thymosin-β4 reverse liver fibrosis?
Currently, studies show that Thymosin-β4 can slow the progression of fibrosis and promote liver regeneration, but its ability to completely reverse fibrosis that has already occurred depends on the stage of the disease and the degree of damage.:
- In the early stages of fibrosis (F1-F2 on the METAVIR scale), Thymosin-β4 can significantly reduce fibrotic tissue and restore normal liver structure due to its regenerative and antifibrotic properties.
- In the late stages of fibrosis (F3-F4, cirrhosis), complete reversal of fibrosis is unlikely, since irreversible structural changes have already occurred in the liver. However, Thymosin-β4 can improve liver function and slow the progression of the disease.
Animal studies:
Preclinical studies in animal models of liver fibrosis have shown that Thymosin-β4:
- Reduces the severity of fibrosis.
- Reduces the level of collagen and other components of the extracellular matrix.
- Improves liver function.
Human clinical trials:
Clinical trials of Thymosin-β4 for the treatment of liver fibrosis in humans are in the early stages. There is not yet sufficient evidence to suggest that the peptide can completely reverse fibrosis in humans. However, the preliminary results are encouraging, and research is ongoing.
Important aspects:
- Integrated approach:
- Treatment of liver fibrosis should be comprehensive and include not only Thymosin-β4, but also other methods, such as:
- Eliminating the cause of fibrosis (for example, treating viral hepatitis, avoiding alcohol).
- The use of antifibrotic drugs (for example, pentoxifylline, silymarin).
- Maintaining a healthy lifestyle (diet, physical activity).
- Treatment of liver fibrosis should be comprehensive and include not only Thymosin-β4, but also other methods, such as:
- Stage of the disease:
- The earlier treatment is initiated, the higher the chances of successful treatment of fibrosis.
- Individual approach:
- The effectiveness of Thymosin-β4 may vary depending on the individual characteristics of the patient and the degree of liver damage.
Conclusion:
Thymosin-β4 has significant potential for the treatment of liver fibrosis due to its antifibrotic, anti-inflammatory and regenerative properties. It can help reverse fibrosis in the early stages, but in the later stages (cirrhosis), its effect is likely to be limited.
- Tissue regeneration: