This test analyzes the genes responsible for the work of detoxification enzymes (phase I and II), which neutralize toxins, drugs, carcinogens and metabolic products. It helps to identify congenital disorders that increase the risk of chronic diseases, intoxication, and drug intolerance.
What genes are being tested?
- Phase I (Cytochrome P450):
- CYP1A1, CYP1B1 – metabolism of carcinogens (for example, benzopyrene from tobacco smoke).
- CYP2D6 -neutralization of 25% of drugs (antidepressants, beta-blockers).
- CYP2C9, CYP2C19 -metabolism of warfarin, NSAIDs, and antidepressants.
- Phase II (conjugation):
- GSTM1, GSTT1 – deactivation of toxins and free radicals (if fission occurs, there is a risk of cancer).
- COMT – destruction of catecholamines (epinephrine, dopamine).
- NAT2 -neutralization of aromatic amines (in case of coffee and tea intolerance).
Why do I need this test?
- Risk assessment:
- Oncology (with weak detoxification of carcinogens).
- Chronic intoxication (heavy metals, pesticides).
- Drug intolerance (for example, slow metabolism of CYP2D6 → codeine poisoning).
- Hormonal disorders (COMT affects estrogens and dopamine).
- Personalized recommendations:
- Selection of safe medications and doses.
- Diet correction (for example, for NAT2 polymorphisms, smoked products are excluded).
- Appointment of antioxidants (for GST defects).
Symptoms of detoxification disorders
With weak enzyme activity (accumulation of toxins):
- Chronic fatigue, headaches.
- Allergies, skin rashes (eczema, acne).
- Intolerance to alcohol, coffee, and medications.
- Increased risk of cancer (especially lung, breast, and bladder).
- Hormonal failures (PMS, endometriosis — with slow COMT).
With hyperactivity of enzymes (rapid breakdown of useful substances):
- Low effectiveness of painkillers or antidepressants.
- Vitamin D deficiency (due to accelerated CYP2R1 metabolism).
- Lack of dopamine (with rapid COMT — > anxiety, ADHD).
Norms in analysis
A genetic test has no ‘norms’ in the traditional sense. The result shows the type of polymorphism:
- Fast metabolism (high enzyme activity).
- Slow metabolism (risk of accumulation of toxins).
- Normal type (optimal function).
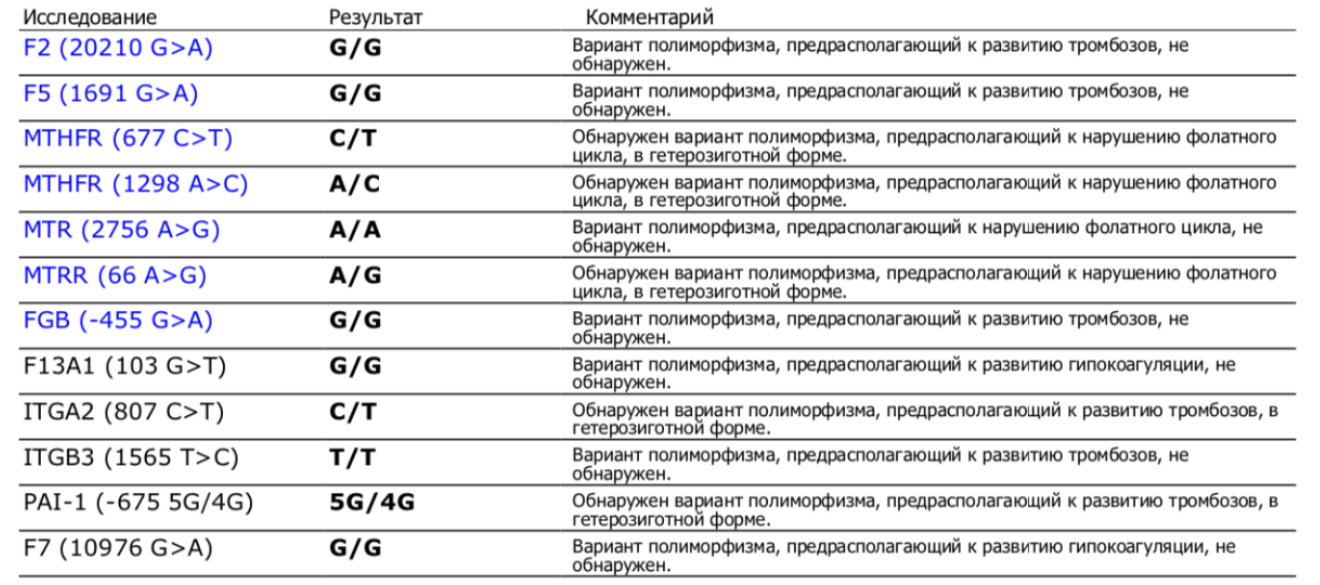
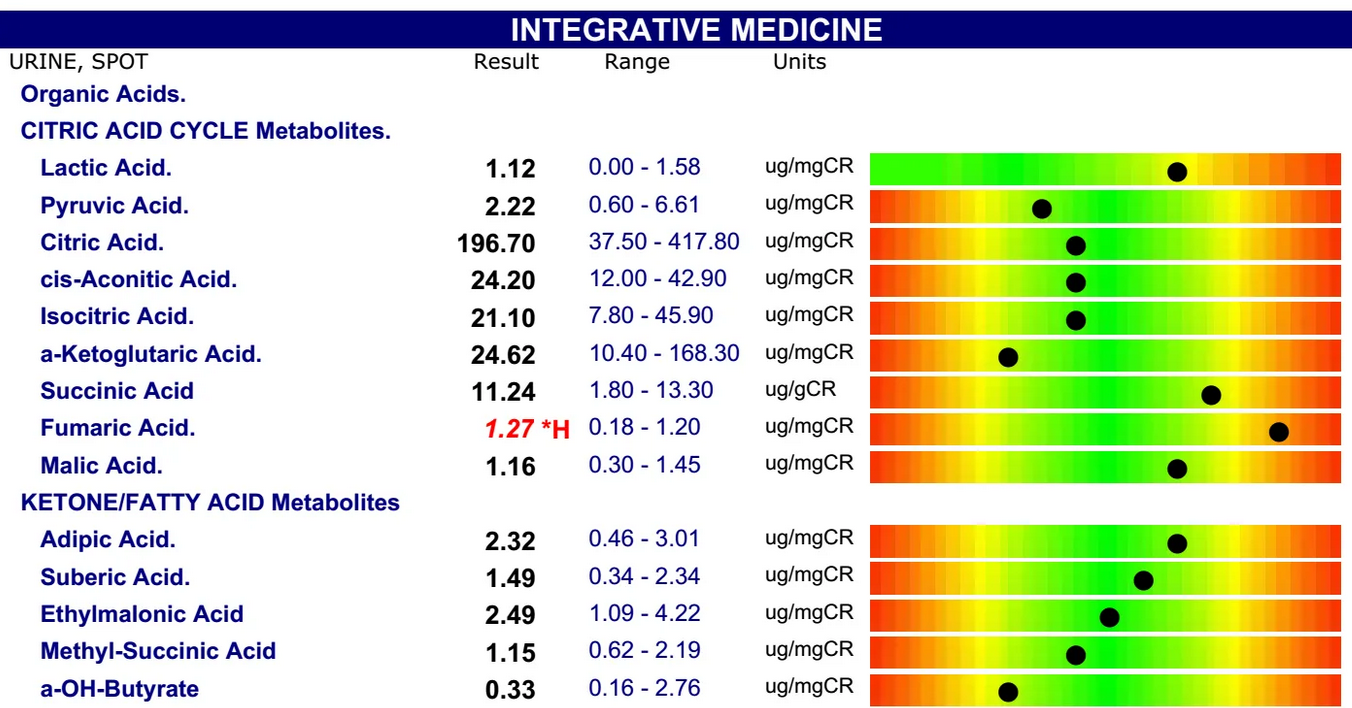
Sample result:
What should I do when detecting polymorphisms?
- Avoid toxins:
- Abstaining from smoking and alcohol.
- Restriction of smoked products (at NAT2).
- Use of eco-friendly cosmetics.
- Detoxification Support:
- Glutathione (for GST defects).
- Magnesium, B12, folate (for the work of cytochromes).
- Indole-3-carbinol (for slow COMT and high estrogens).
- Personal selection of medicines:
- For’ slow metabolizers ‘ – lower doses.
- For’ fast ‘ – alternative drugs.
Examples of the impact of polymorphisms
| The gene | Polymorphism | Effects | Recommendations |
|---|---|---|---|
| CYP2C19 | 2/2 | Ineffectiveness of clopidogrel | Replacement with ticagrelor |
| COMT | Val158Met | Anxiety, low dopamine | SAM-e, magnesium |
| GSTM1 | Deletion | Lung cancer risk | Curcumin, selenium |
Conclusion
- The detoxification polymorphism test identifies individual risks of diseases and drug reactions.
- Symptoms of violations: chronic intoxication, drug intolerance, hormonal disorders.
- ‘Norms’ is the definition of the type of metabolism (fast/slow).
- Correction: diet, antioxidants, personalized pharmacotherapy.
The test is especially useful when:
- Unexplained chronic symptoms.
- Family history of oncology.
- Frequent side effects from medications.